Unlock High Catalyst Activity in Steam Reforming: Syamcat's Breakthrough
- Share
- publisher
- Lawrence
- Issue Time
- Jun 6,2024
Summary
For steam reforming, noble metal catalysts show high activity due to strong methane activation and hydrogen evolution abilities, but their high cost hinders industrial-scale use. Among non-noble metal catalysts, Ni, Co, and Fe are key components, ranked in activity as Ni > Co > Fe. Nickel-based catalysts, with their comparable activity to noble metals and much lower cost, are especially promising for industrial applications.
Key Factors Affecting Catalyst Activity
The loading amount of the metal active components is a crucial factor affecting catalyst activity. Ni and Co catalysts require relatively high loadings. Ruckenstein et al.[1] studied the effect of Ni content on Ni/Al2O3 catalysts: they found that when the Ni content was 1%, the catalyst had a very high initial conversion rate and CO yield. When the Ni content was 9%, a strong interaction between NiO and the Al2O3 support led to the formation of highly dispersed two-dimensional surface compounds, thus achieving high catalytic activity.
Importance of Catalyst Supports
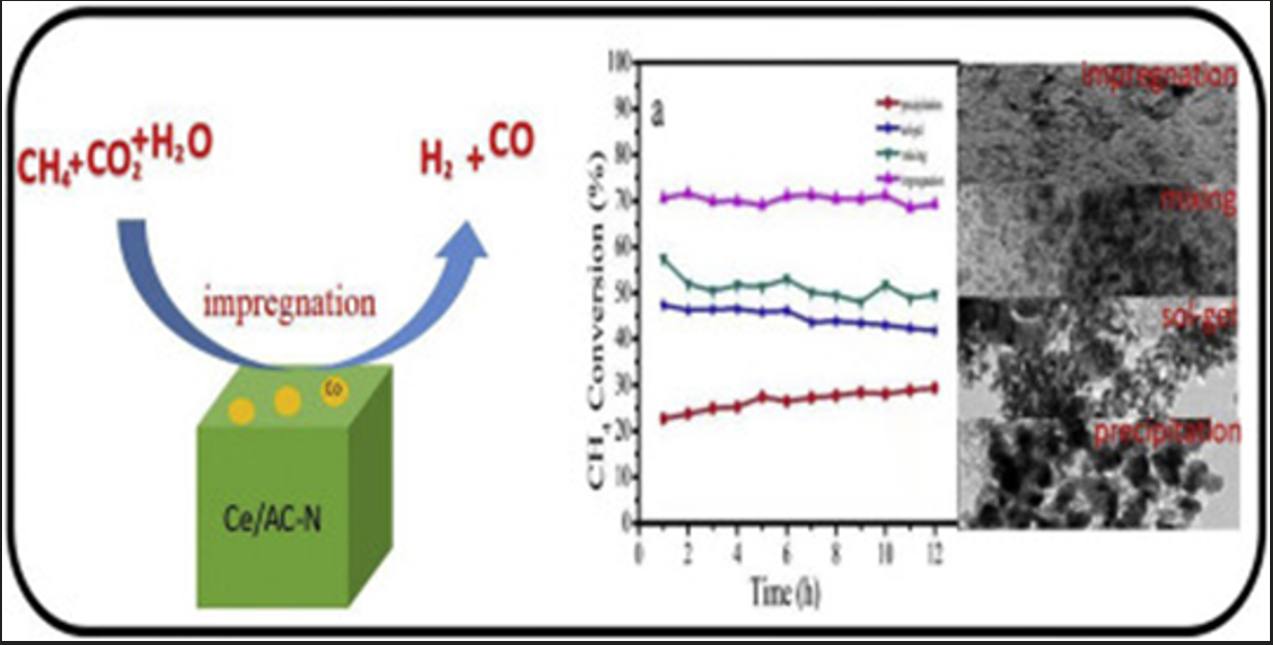
Catalytic reforming requires high temperatures to obtain good catalytic activity, so the chosen reforming catalyst support must possess excellent thermal stability. Therefore, supports such as Al2O3, SiO2, MgO, CaO, TiO2, ZrO2, silica, Si3N4, foam ceramics, rare earth oxides, and some composite metal oxides like MgO-Al2O3, ZrO2-Al2O3, CaO-SiO2, and high-melting-point substances like molecular sieves are generally selected as catalyst supports. Deng Cun et al.[2] believed that the differences in structural properties of supports and the varying interactions between metals and supports might be the main reasons for the differing activities of supported Ni catalysts. The stronger the interaction between the metal and the support, the harder it is to reduce the catalyst, leading to greater dispersion of the metal on the surface after reduction and stronger resistance to sintering during the reaction process. Jiang Xuanzhen et al.[3] suggested that the activity and selectivity of a catalyst are related to the relative alkalinity of the support, which should have a suitable ability to dissociatively activate the reactants.

Syamcat's Advanced Support Technology
Syamcat 's R&D team has been deeply engaged in support research for many years and has developed special support technologies. The advancements in support technology have enabled the company's steam reforming catalysts to achieve not only high activity but also significantly improved catalytic efficiency, all while ensuring cost-effectiveness.ontent
Conclusion
In summary, the improvement of steam reforming catalyst activity can be significantly influenced by the choice of active metal components and the type of support used. Vant Company's innovative support technology represents a major advancement in this field, providing high-performance, cost-effective solutions for steam reforming applications.
For more information on Syamcat's advanced catalyst support technology and how it can benefit your steam reforming processes, contact us today or visit our product page.
References
1. Ruckenstein E, Hu Y H. Role of support in CO2 reforming of CH4 syngas over Ni catalysts[J]. Catalysis, 1996, 162: 230-236.
2. Lu Yong, Deng Cun. Reforming of methane and carbon dioxide to synthesis gas over supported nickel metal catalysts[J]. Journal of Catalysis, 1996, 17(003): 212-216.
3. Jiang Xuanzhen. "Partial oxidation of methane to syngas: II. The effect of support." (1995).